Molecular Section
- Home
- Lab Sections
- Molecular Section

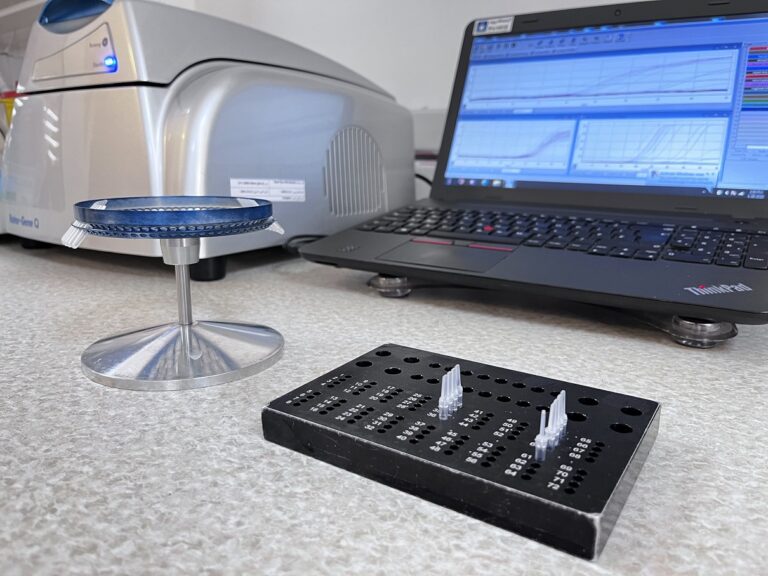
Since Payvand laboratory policy has always been to improve service quality and to be up-to-date, the molecular section of this laboratory also equipped with the latest molecular methods in the diagnosis, control of malignant and non-blood malignant diseases, bacterial infectious diseases, viruses, Fungi, coagulopathies and thrombophilia disorders. Currently, more than a hundred different molecular tests will be done on the DNA and RNA of patients from different samples including blood, serum, plasma, cerebrospinal fluid, pathological blocks, peripheral blood, and bone marrow.
Our Lab is using the verity of diagnostic’s methods such as PCR, RFLP, RT-PCR, Allele-specific PCR, Reverse dot blot, etc. with advanced and automated devices in order to provide the most accurate result at the earliest time.
It should be noted that Payvand Clinical & Specialty laboratory has been recognized as a referral diagnostic center for the BCR-ABL Quantitative & BCR-ABL Qualitative Tests which is ordered to evaluate the response to treatment of CML patients.
To maintain the quality and provide assurance and approval for the Molecular tests which run at Payvand Laboratory, we received an International certificate from the Ras Expert Laboratory LMU in Germany for two consecutive years for the KRAS / NRAS / BRAF tests and known as one of the approved testing centers. In addition, The wide range of diagnostic and screening tests can be done for diseases such as Synovial sarcoma, Ewing’s Sarcoma, Rhabdomyosarcoma (Alveolar type), ALL, CML, AML, coagulation panels, and rearrangement assay in IGH, IGK, TCR-δ, TCR-γ For prognosis of clonality of lymphoma and MRD in ALL as a prognostic marker, evaluation of clonality, celiac, colorectal cancer panel, myelofibrosis, polycythemia vera, neuroblastoma, hypereosinophilia syndrome, evaluation of drug resistance in patients with CML and lymphocytic cancer (MANTEL CELL) Lymphomas, hemochromatosis as well as determination of drug dosage of warfarin, etc. at Payvand Clinical & Specialty laboratory.
Important indicators of Molecular Diagnosis section are:
- Establishing a quality management system in the framework of national and ISO standards (ISO 10002 + ISO 9001)
- Continuous monitoring of the quality of the tests with respect to internal and external quality control (in some cases)
- The speed at which molecular testing is performed so that in the case of an emergency, the test time reaches less than 24 hours
- Significant variation in molecular tests that are unique in the country and even in the countries of the region
- Employing staff with a minimum degree of postgraduate degree in relevant fields with the necessary knowledge and experience
- Use of the newest equipment and technology in the world
- Co-authored by two international pharmaceutical companies to monitor and test the two malignancies (CML) and the KRAS / NRAS mutations



























Follow us in